9F, Zhongrui Jumei Building, 68 Jiuzhang Road, Suzhou Industrial Park, Jiangsu Province
Policies and regulations
newest! According to the National Health Insurance Bureau, 343 drugs are expected to enter the new medical insurance catalog
Recently, the National Health Insurance Bureau issued the "Announcement on the Approval of the Formal Review of the Drug List of National Basic Medical Insurance, Industrial Injury Insurance, and Maternity Insurance in 2022" (hereinafter referred to as the "Announcement").
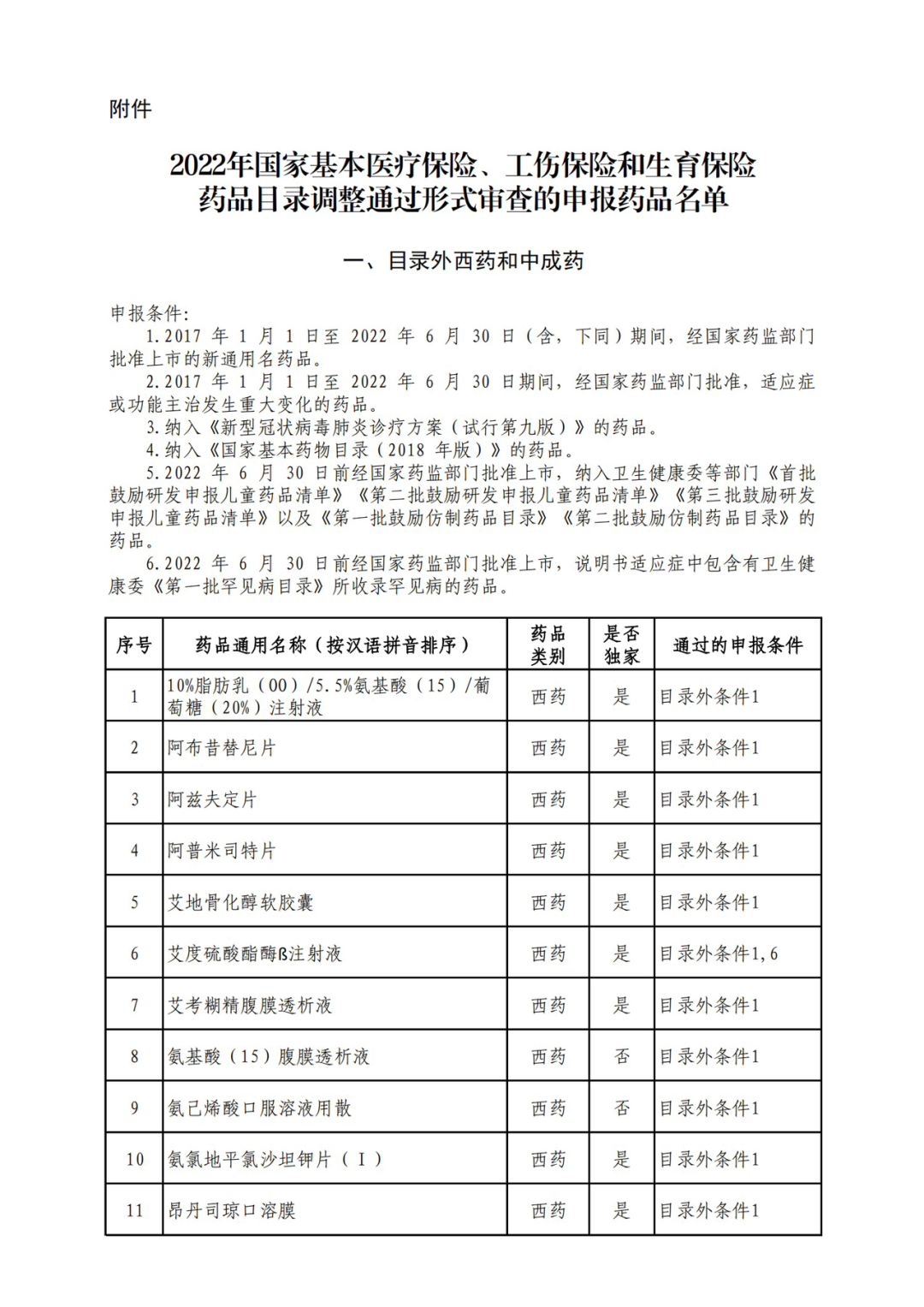
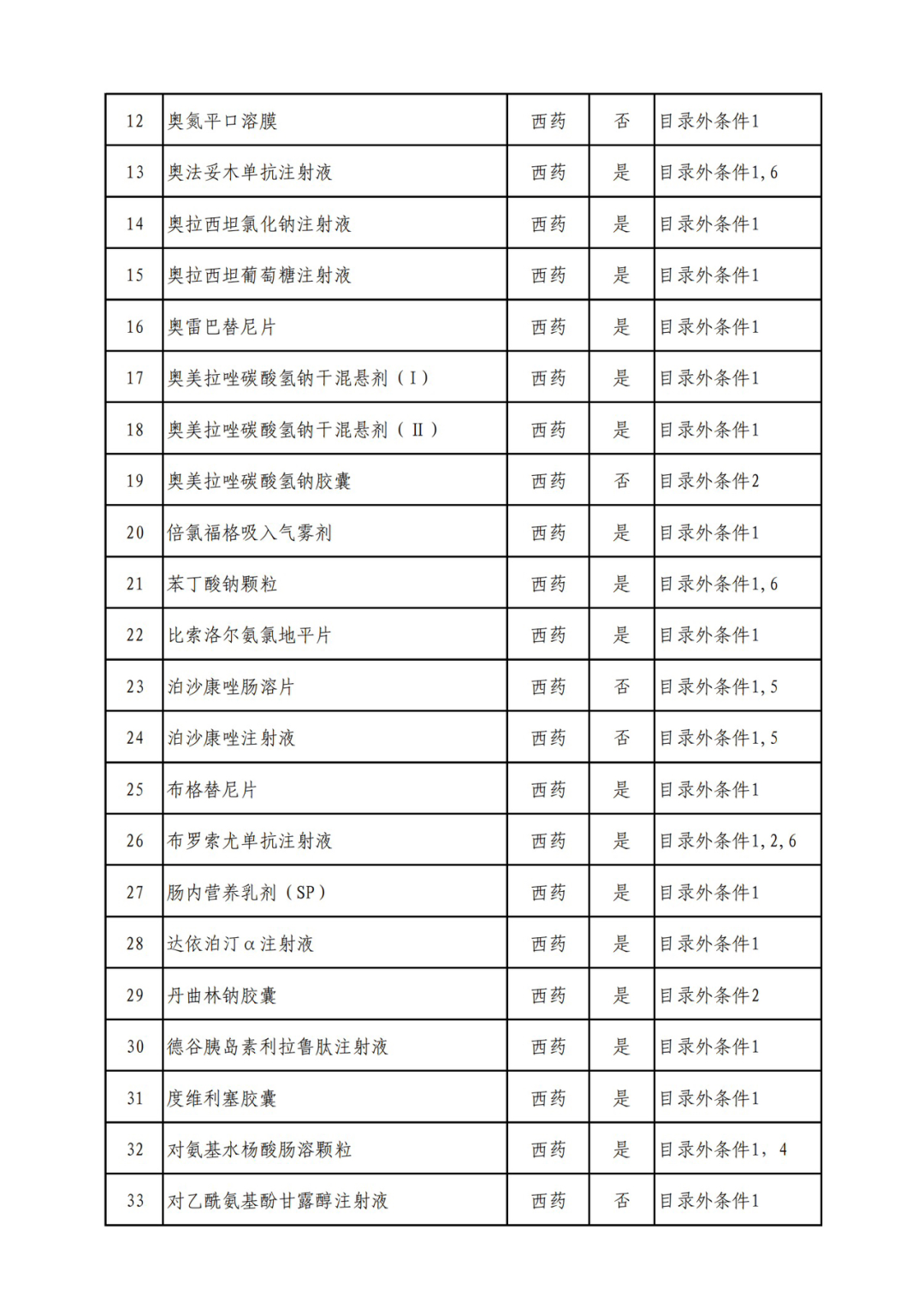
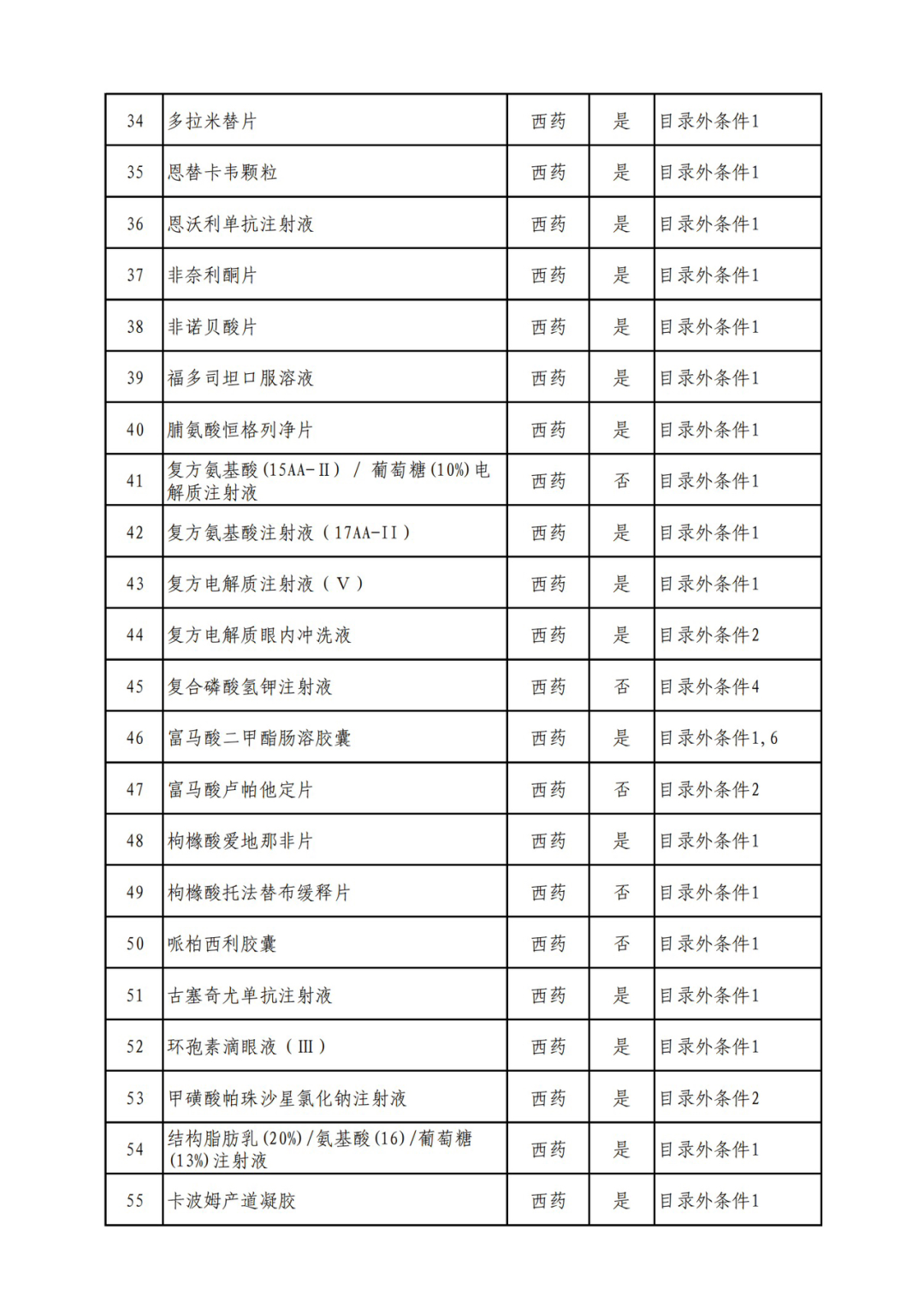
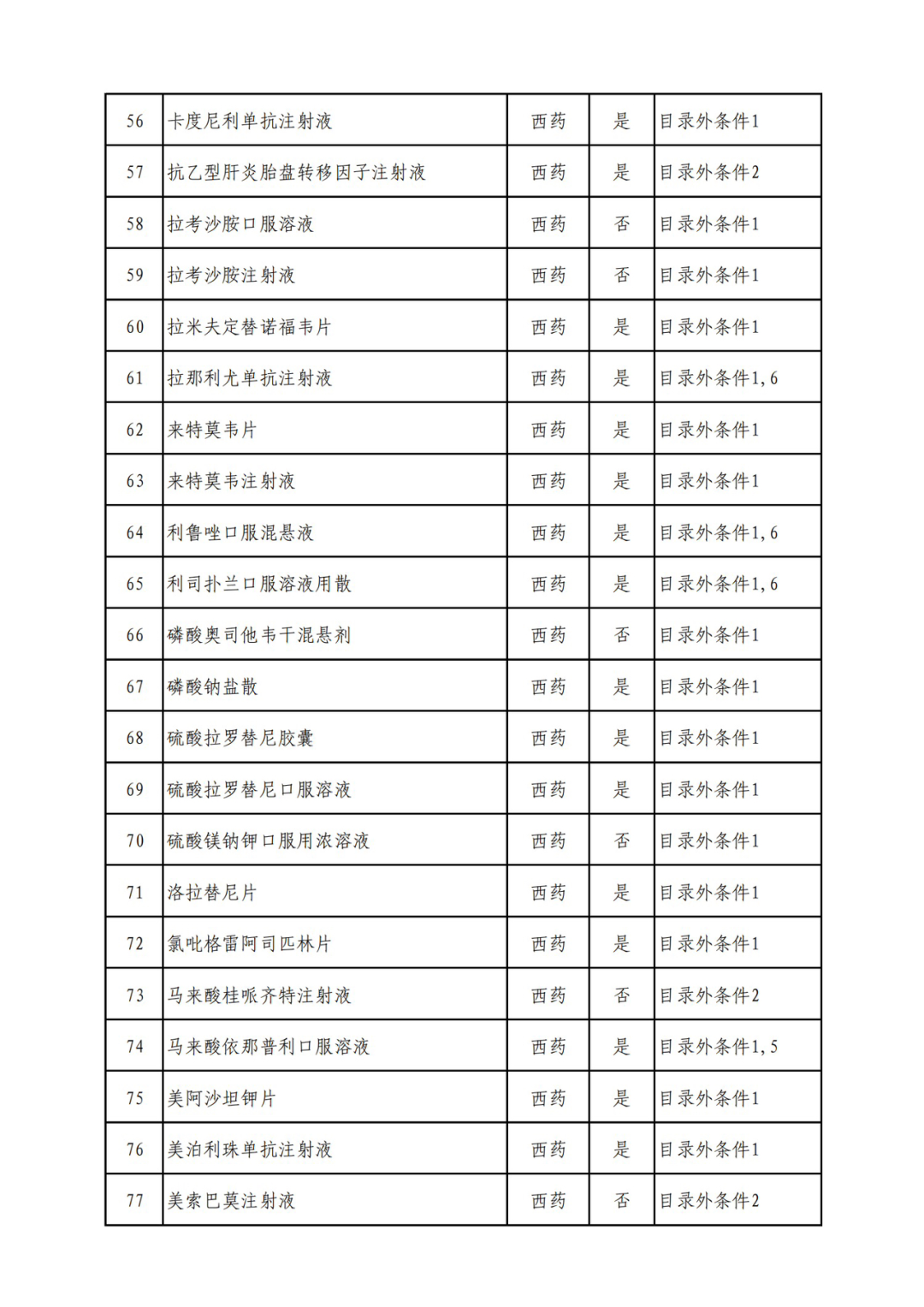
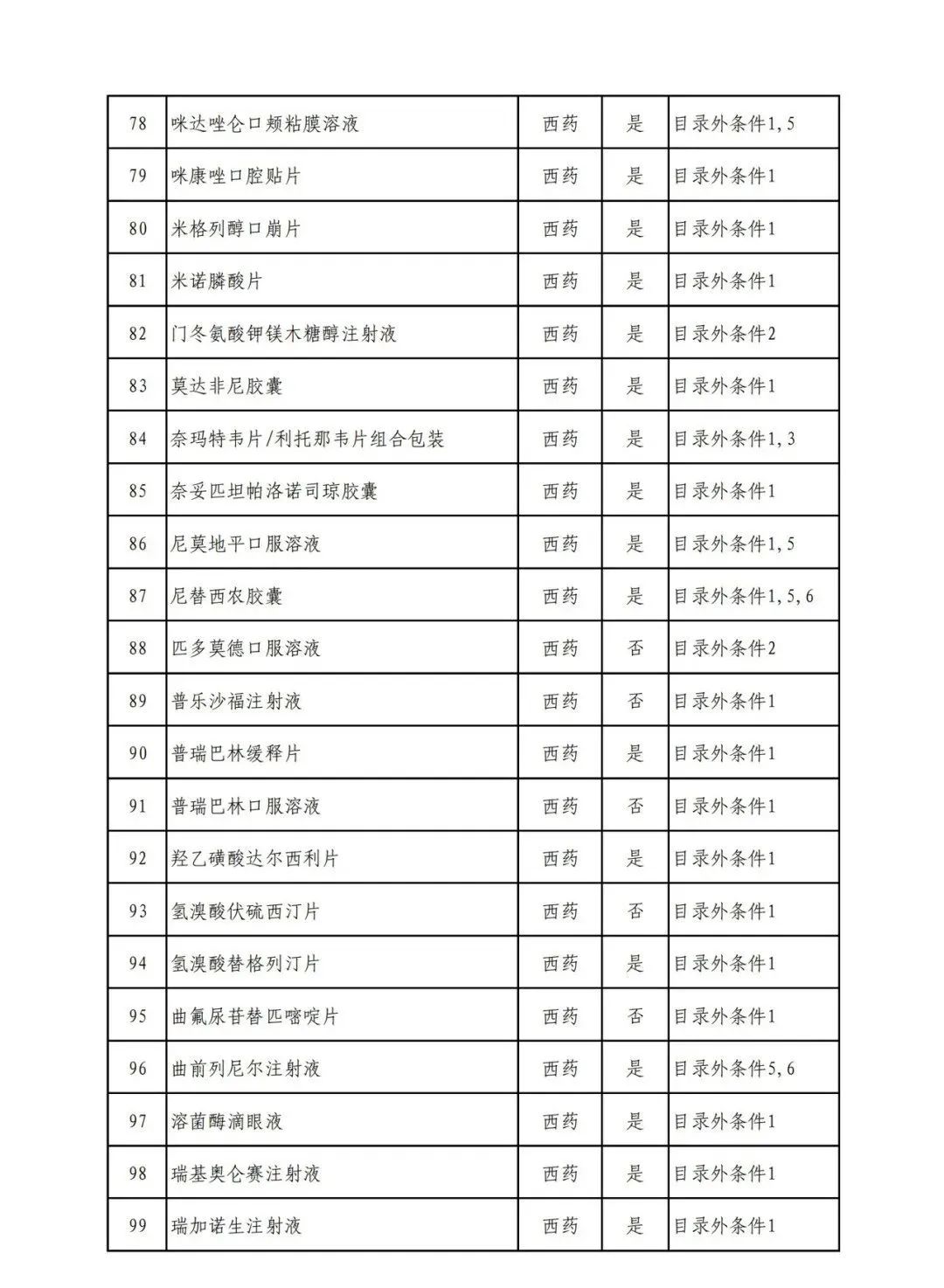
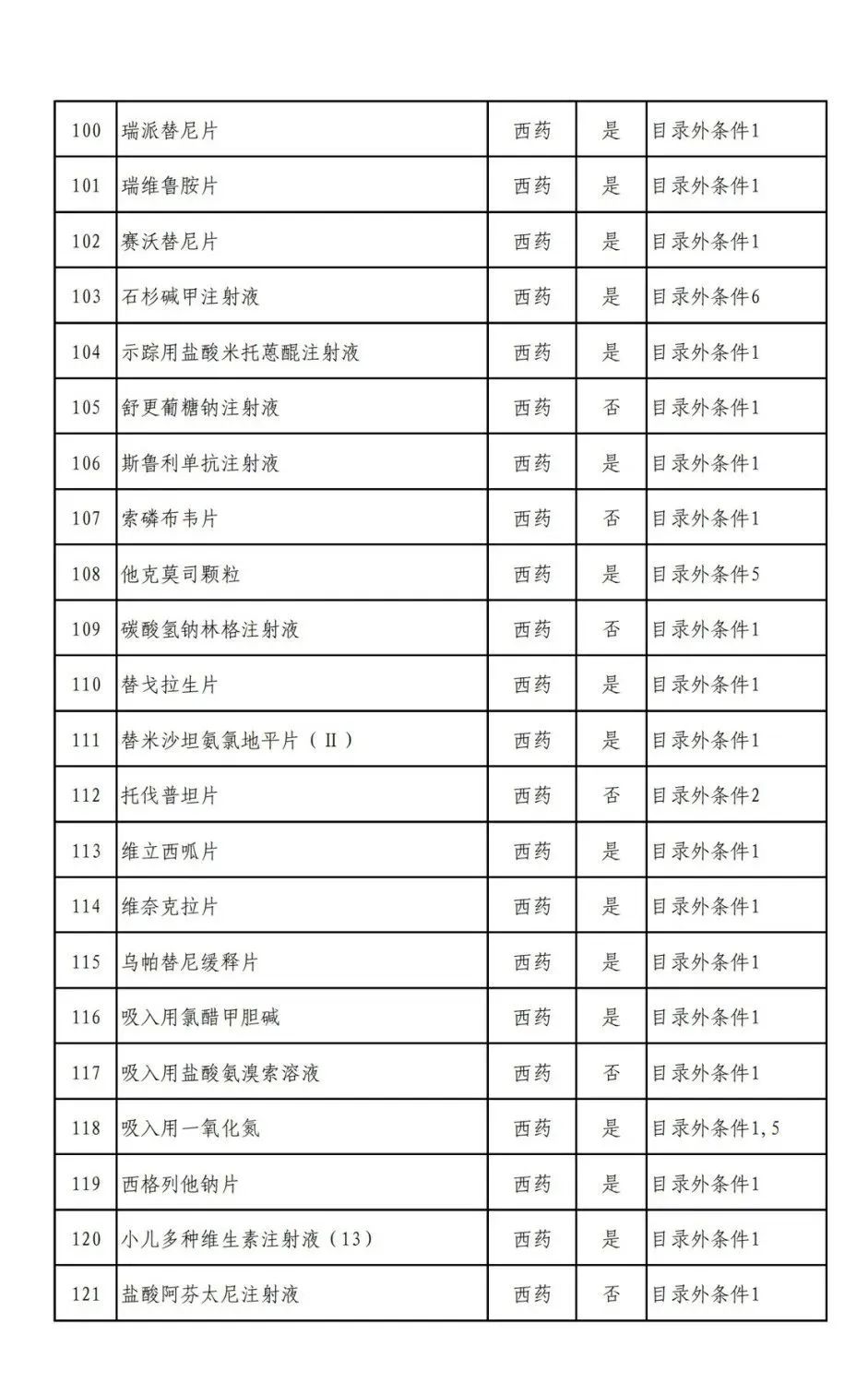
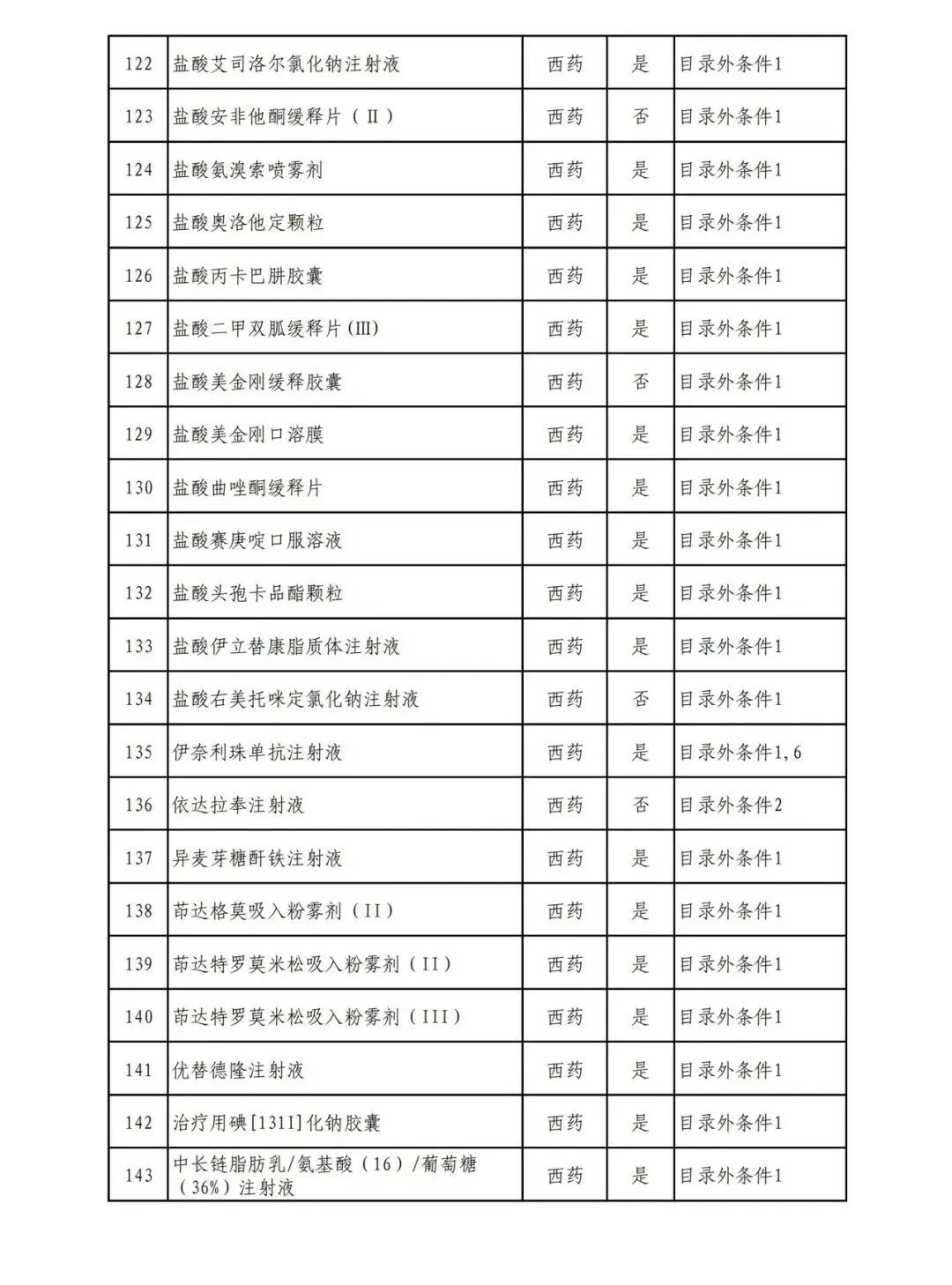
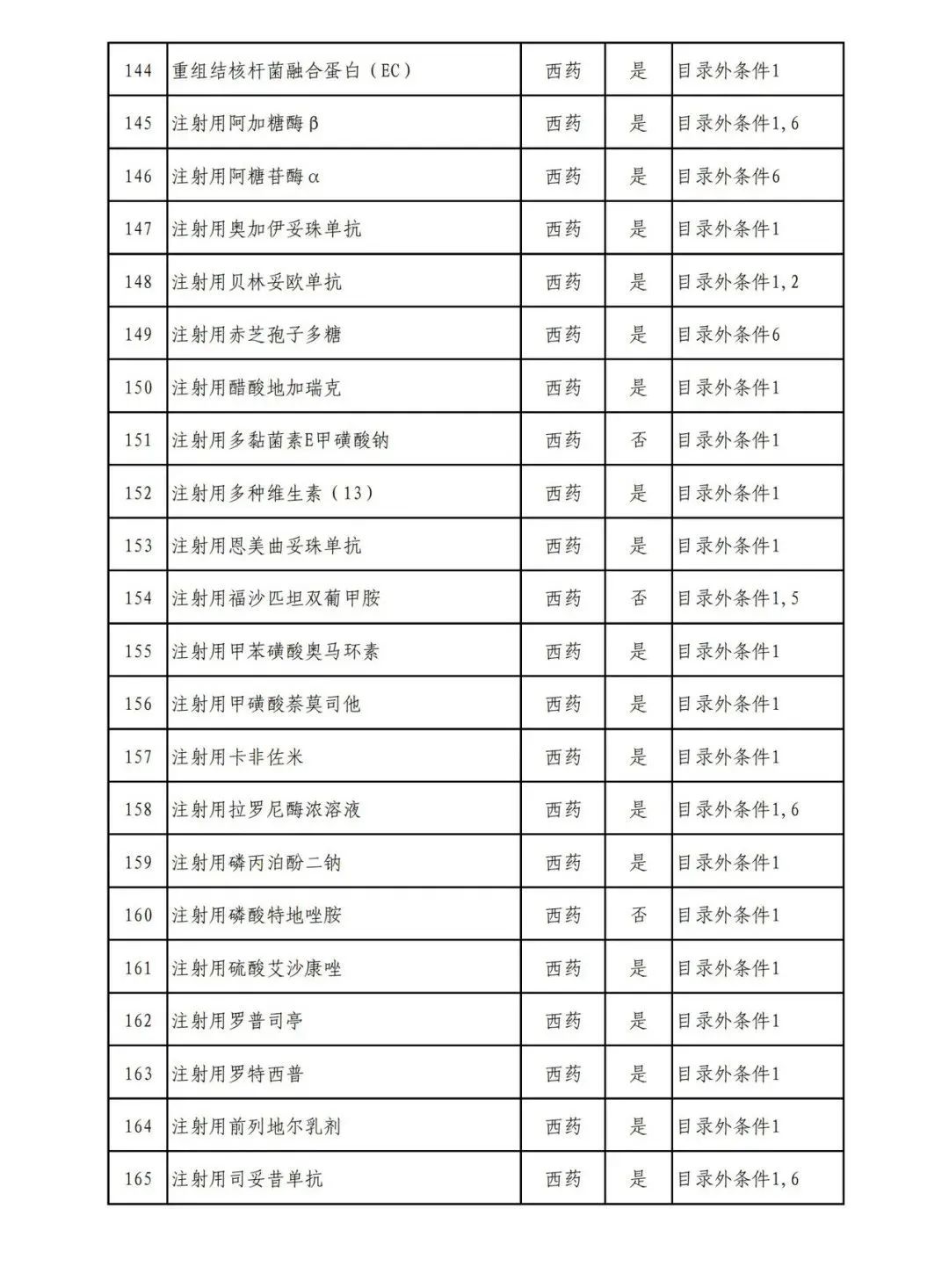
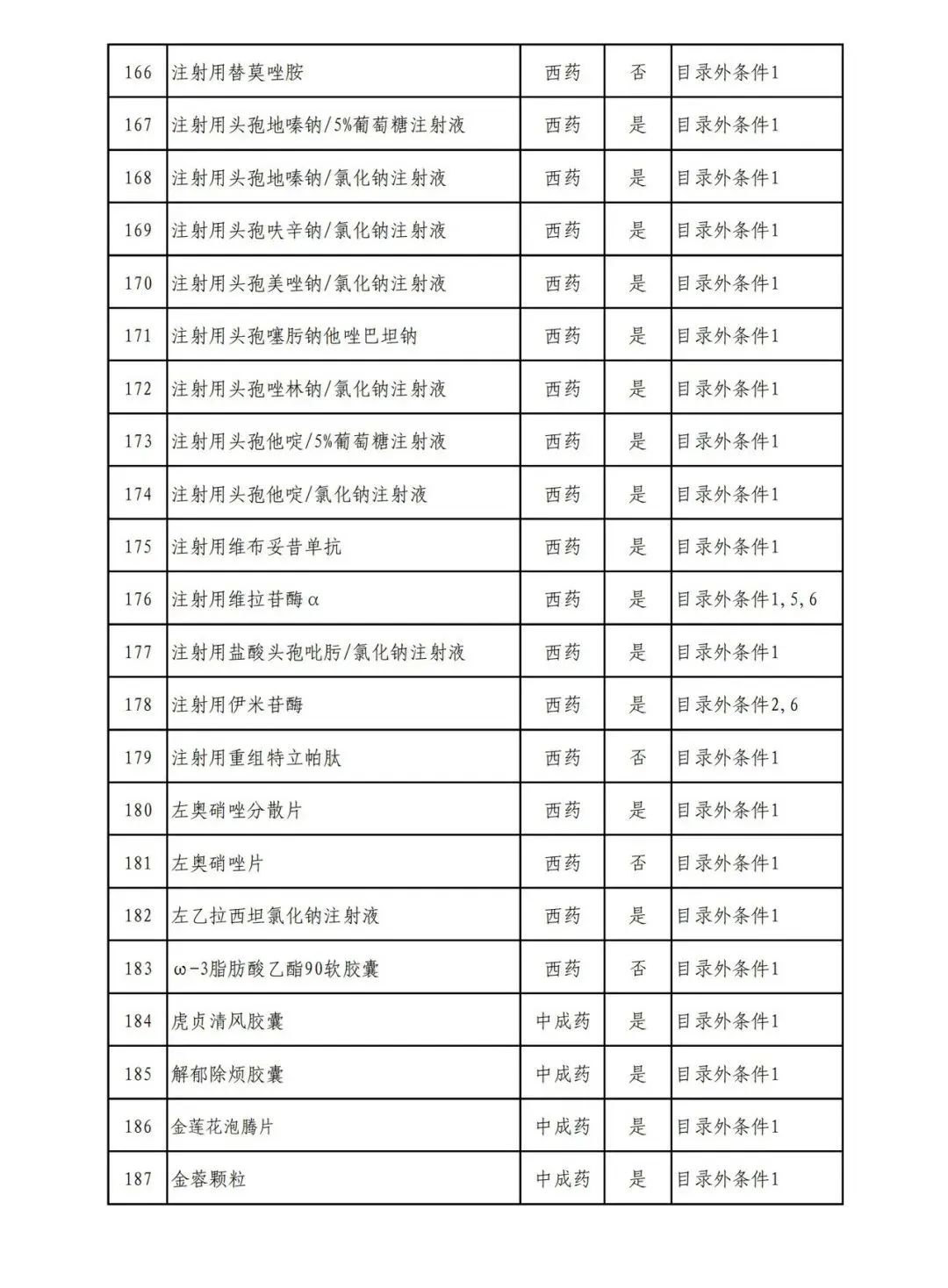
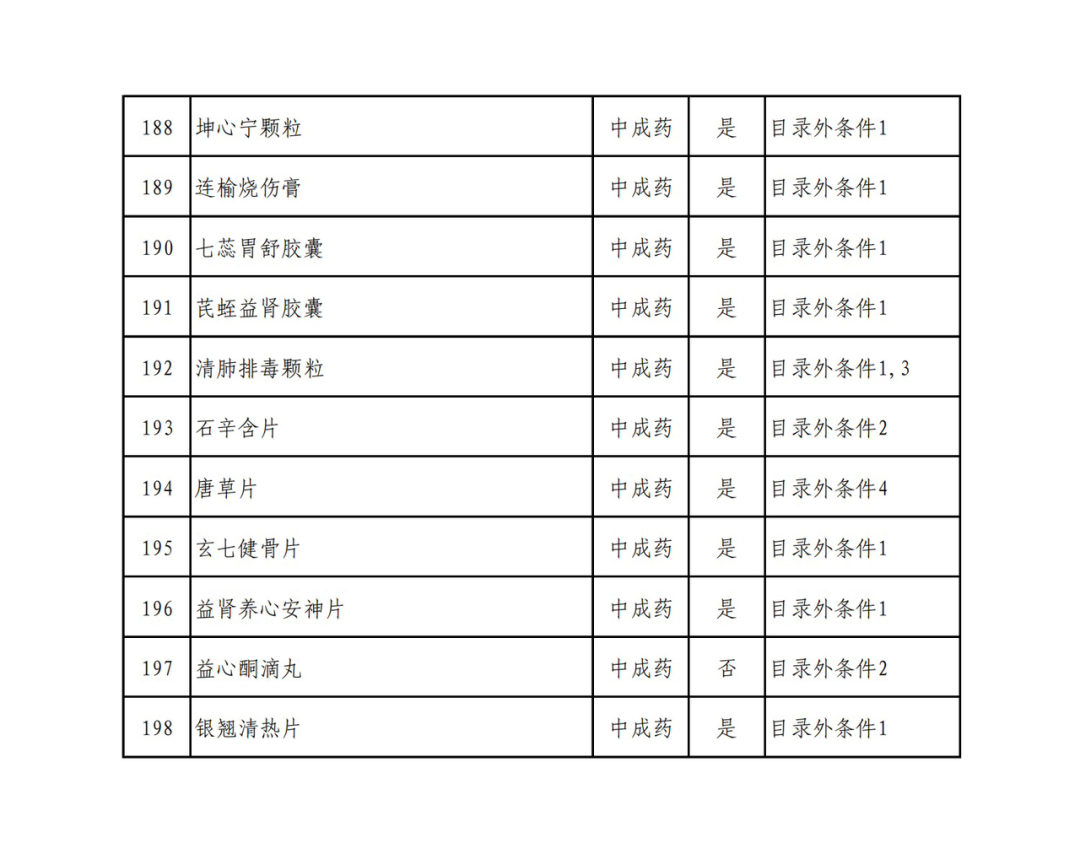
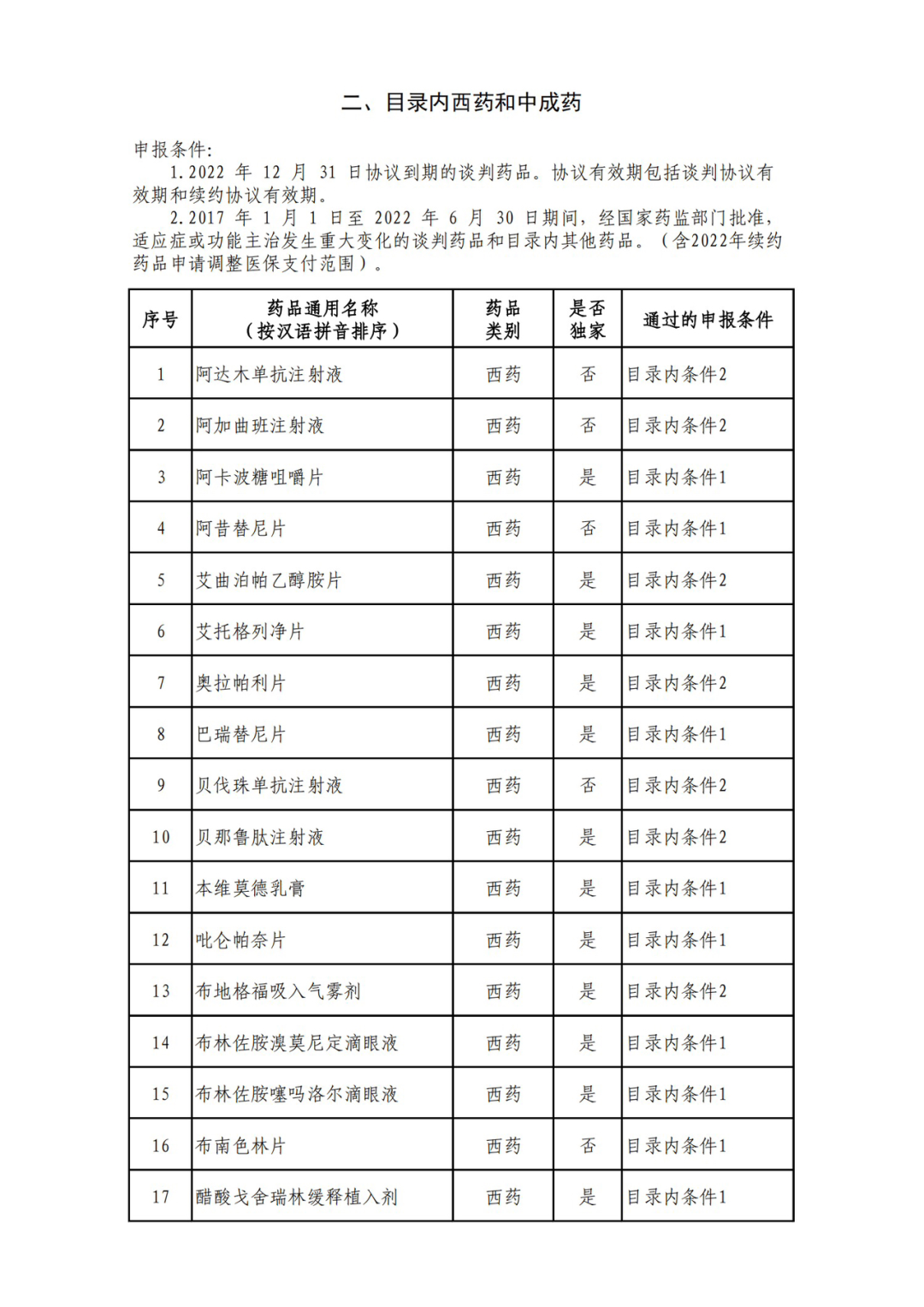
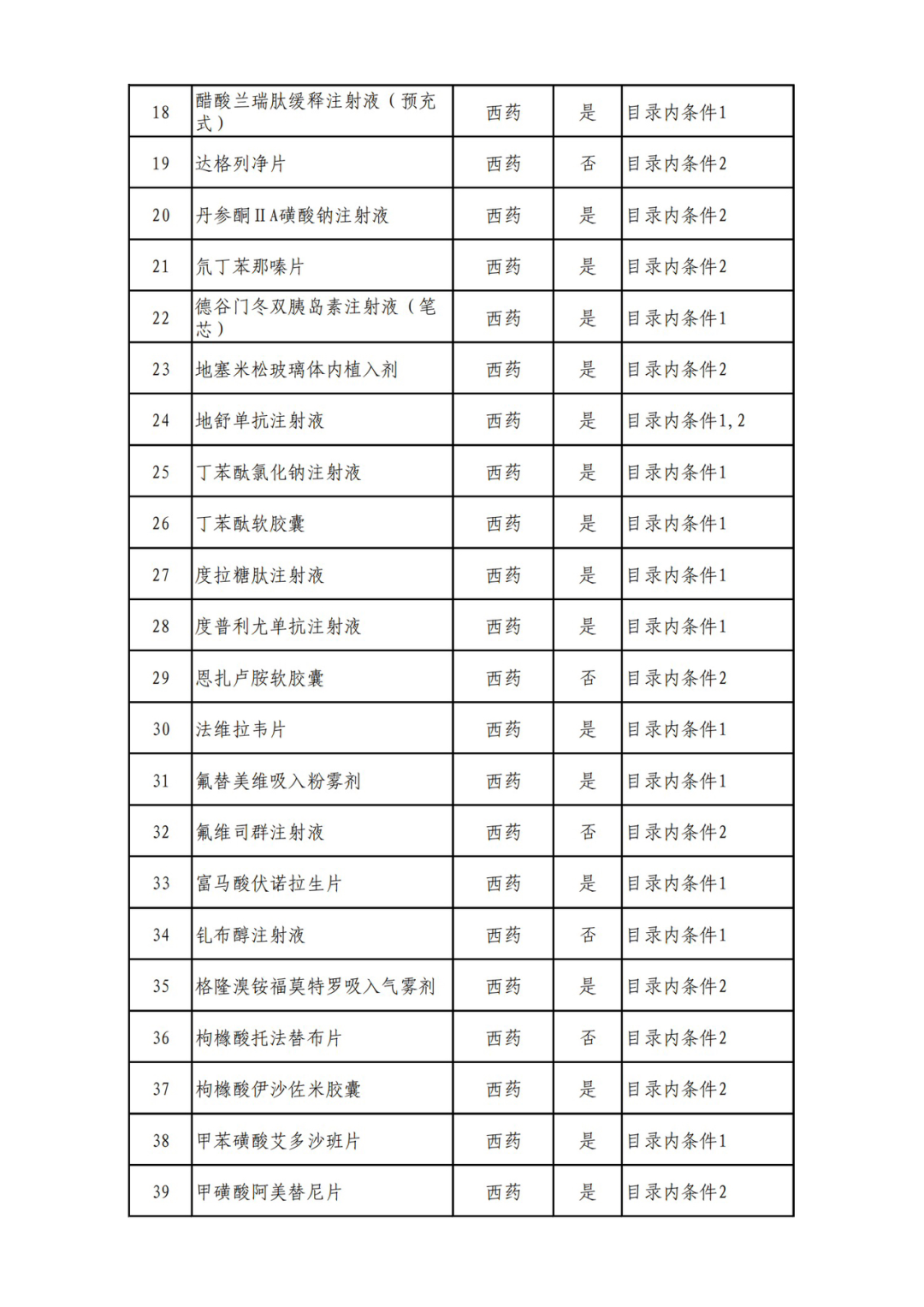
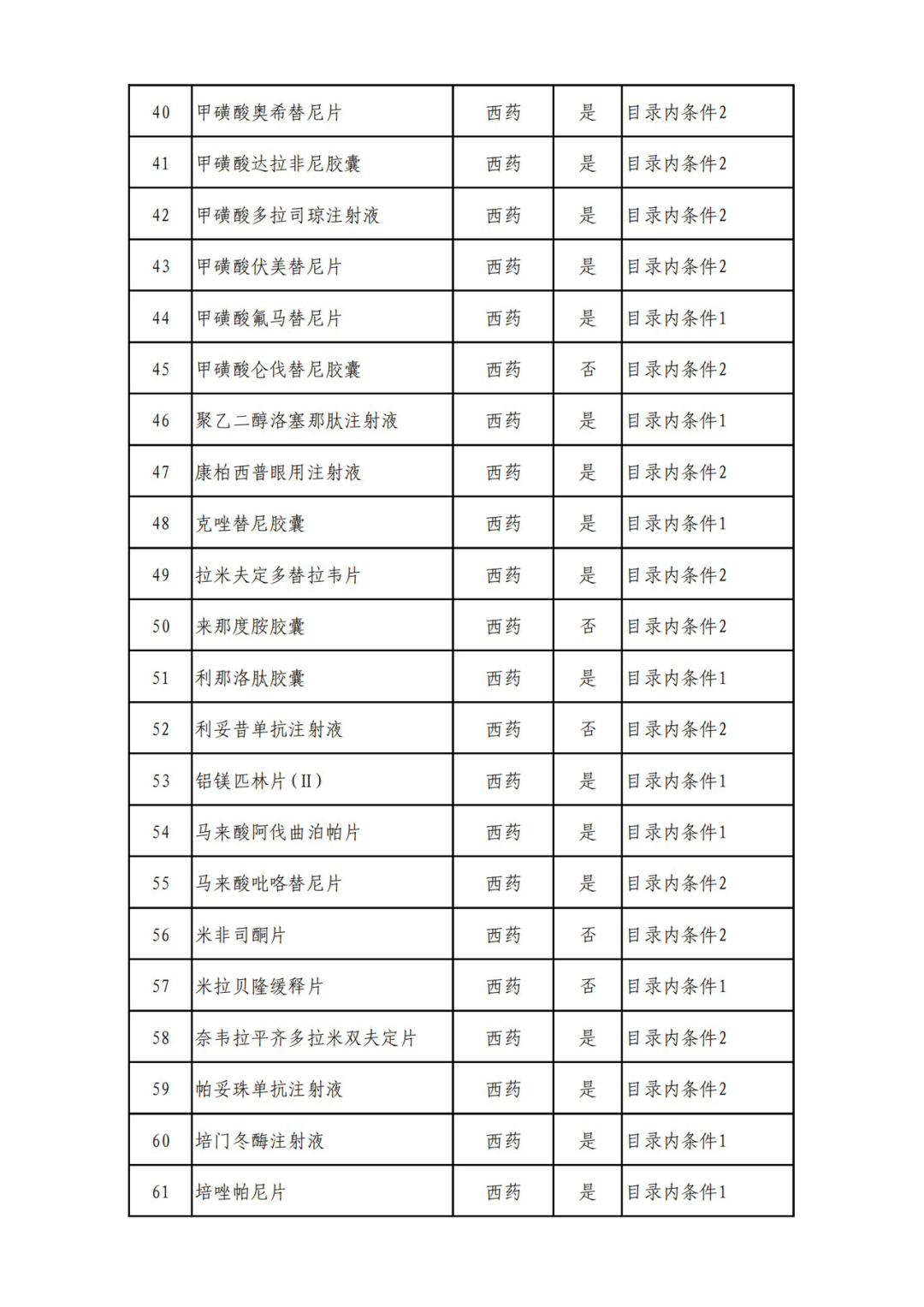
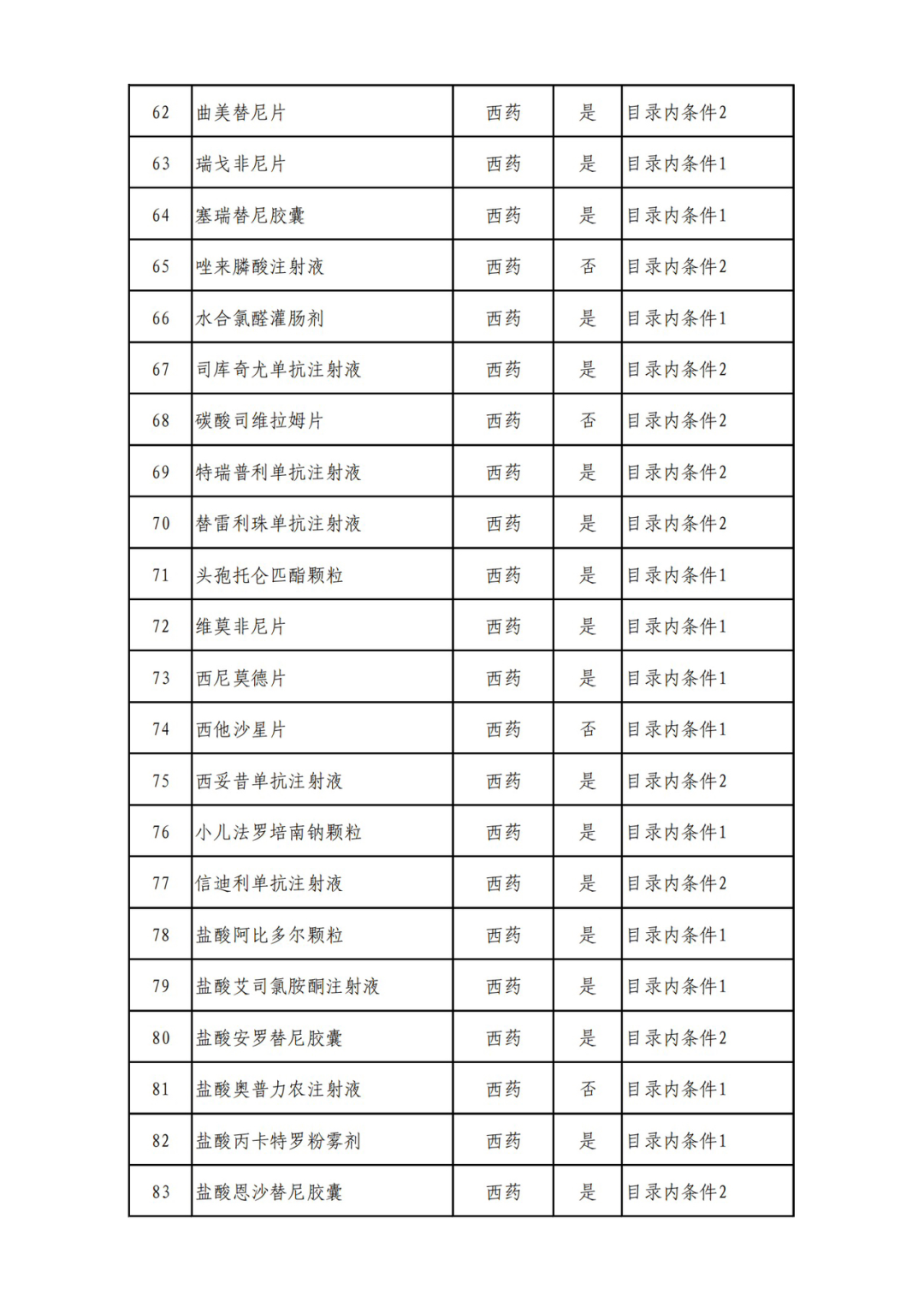
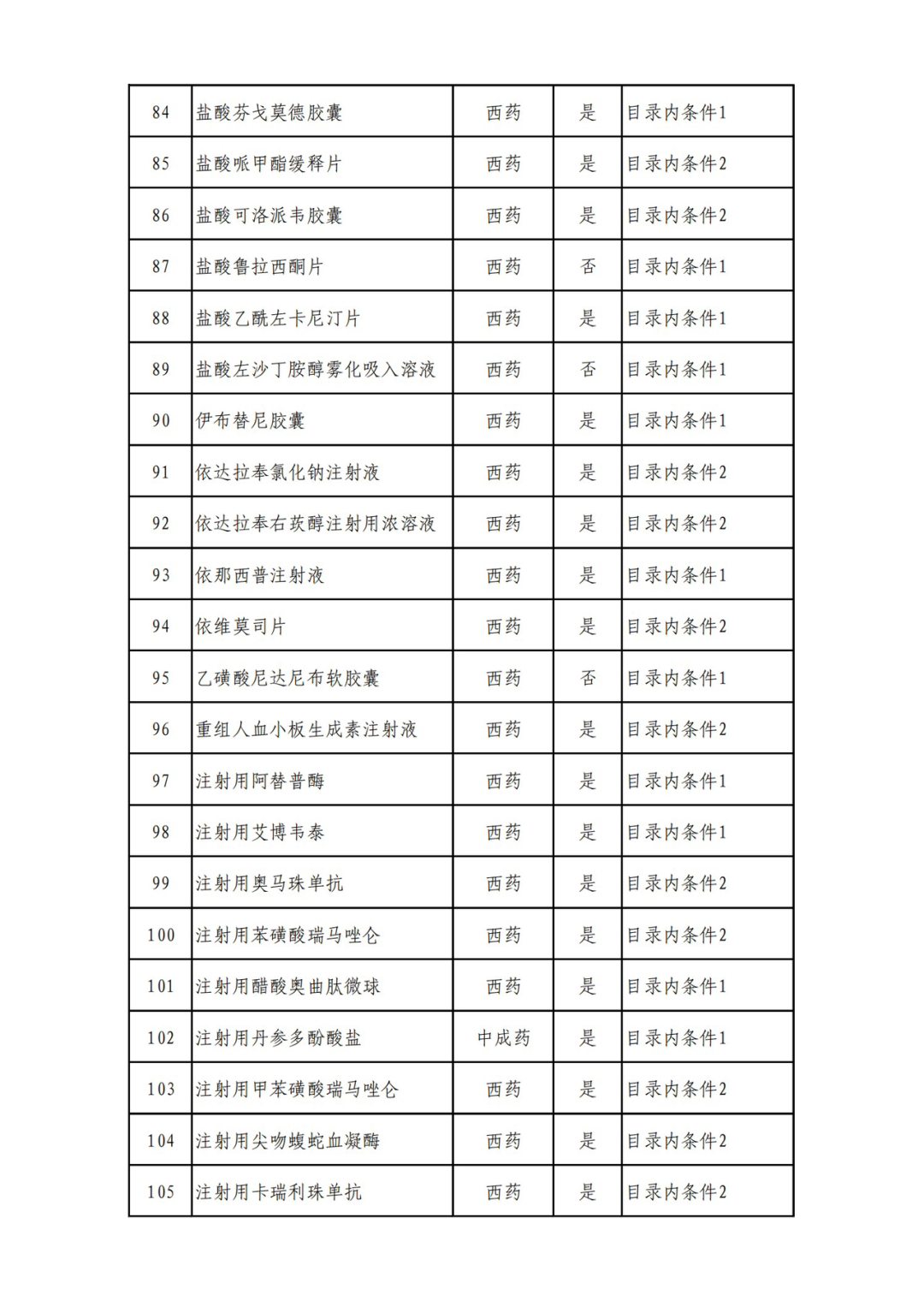
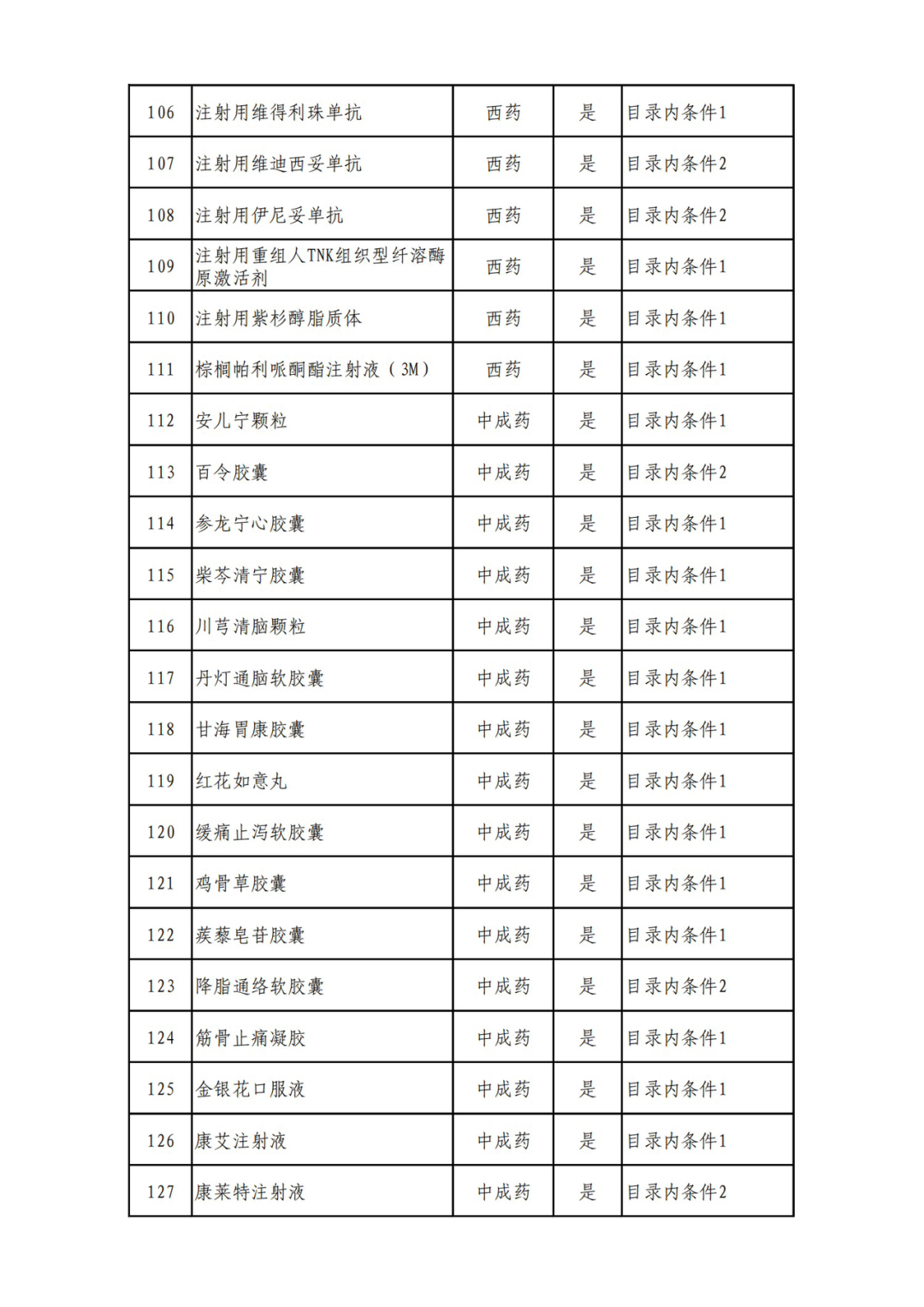
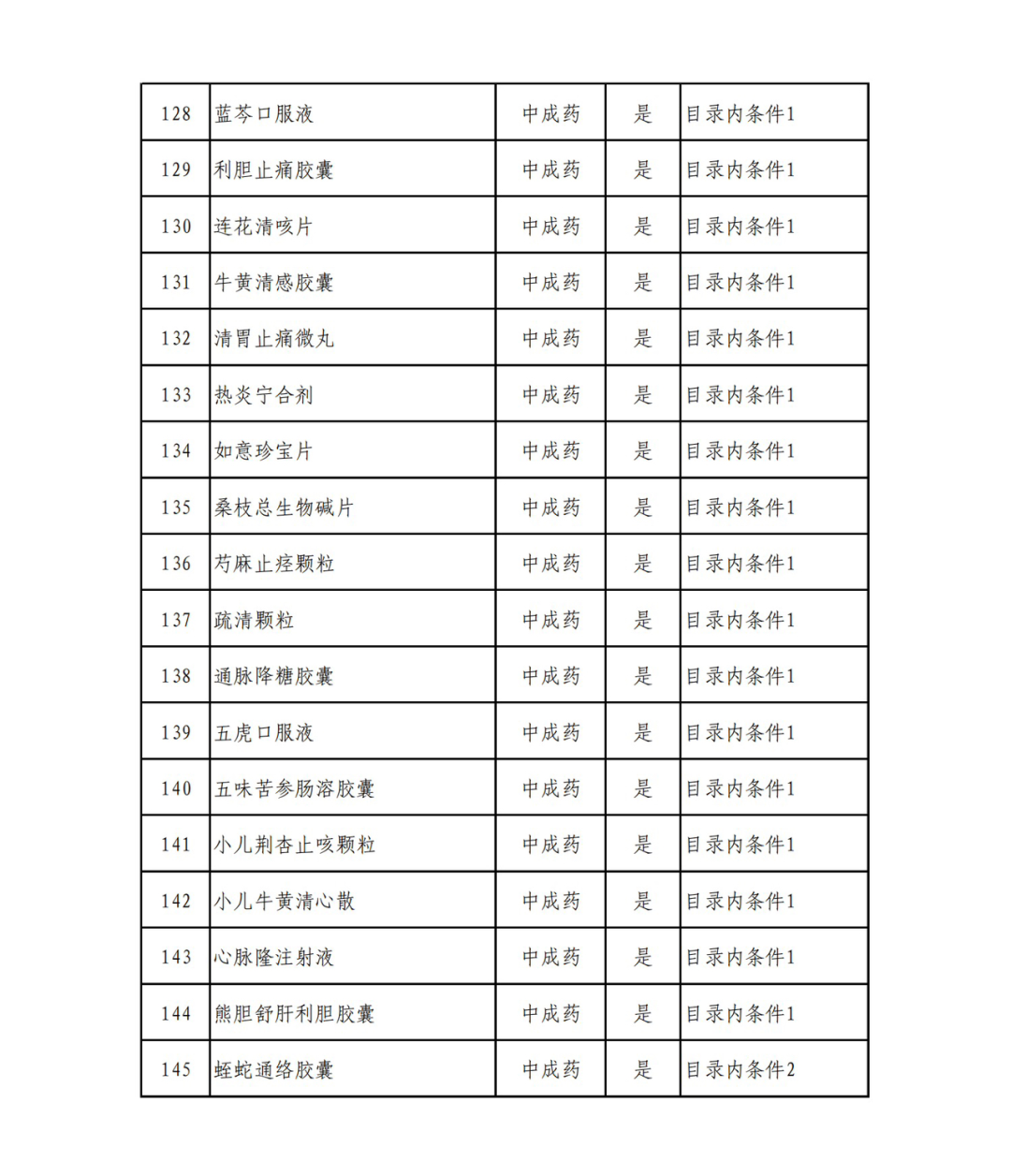
According to the Announcement, there are 198 western medicines and traditional Chinese patent medicines and simple preparations off the list, 145 western medicines and traditional Chinese patent medicines and simple preparations on the list, and a total of 343 drugs have passed the formal examination.
On the evening of September 6, the State Health Insurance Administration issued the Announcement on Publicizing the Drugs and Related Information that have passed the Preliminary Form Examination of the 2022 National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List Adjustment. Upon examination, 344 drugs passed the preliminary form examination, including 199 foreign and traditional Chinese patent medicines and simple preparations, and 145 western and traditional Chinese patent medicines and simple preparations in the list, with a passing rate of 70%.
Based on feedback from various parties, the National Health Insurance Bureau reviewed the relevant drugs and revised the results according to procedures.
Specifically, there are 183 chemical drugs out of the list (one less "metronidazole and vitamin ginseng vaginal expansion suppository" than before), and 15 traditional Chinese patent medicines and simple preparations; There are 111 chemical drugs and 34 traditional Chinese patent medicines and simple preparations in the catalogue.
It is now September, and from the perspective of timing, the negotiation phase will begin next.
The procedures for adjusting the national drug catalog in 2022 are similar to those of last year, and are divided into five stages: preparation (May June 2022), application (July August 2022), expert review (August 2022), negotiation (September October 2022), and publication of results (November 2022).
The negotiation stage is divided into five steps:
Improve the negotiation/bidding drug submission material template.
Organize the submission of relevant materials according to the company's intentions.
Conduct price measurement and evaluation. Organize measurement experts to conduct evaluation through fund measurement, pharmacoeconomics, and other methods, and provide evaluation opinions.
Strengthen communication. Establish a communication mechanism with enterprises, register their opinions, suggestions, and appeals, and respond in a timely manner. Conduct face-to-face communication with enterprises on the ideas and priorities of drug measurement and evaluation, and solve problems in a timely manner.
Conduct on-site negotiation/bidding. Organize on-site negotiation/bidding based on the evaluation opinions, determine a unified national medical insurance payment standard, and clarify management requirements simultaneously.
Among them, measurement experts are composed of experts in medical insurance management, pharmacoeconomics, and other aspects recommended by local medical insurance departments and relevant units. It is divided into a fund measurement group and a pharmacoeconomics measurement group, which provide evaluation opinions on negotiated drugs from two aspects: the impact of medical insurance funds and the pharmacoeconomics evaluation.
The negotiation experts are composed of representatives from the medical insurance department and relevant experts, who are responsible for on-site negotiation/bidding with the negotiating drug companies.
After four years of practical work, the medical insurance catalog has established a dynamic adjustment mechanism based on a regular adjustment cycle of one year. The time interval for catalog adjustment has been continuously shortened and adjustments have become more flexible.
Therefore, drugs with significant clinical value will be quickly adjusted to enter the catalog, and drugs with auxiliary drugs and poor drug economy will be transferred out, speeding up the replacement of medical insurance funds.
COVID-19 oral medicine, innovative tumor medicine All included
From the list of varieties, 198 drugs out of the list include COVID-19 oral drugs, innovative tumor drugs and rare disease drugs.
The National Health Insurance Bureau said that, in the context of the normalization of epidemic prevention and control, since the first implementation of the independent declaration system of medical insurance drug catalog enterprises in 2020, the National Health Insurance Bureau has attached great importance to COVID-19 treatment drugs, taking "drugs included in the Diagnosis and Treatment Plan for novel coronavirus Pneumonia" as one of the application conditions. A number of COVID-19 therapeutic drugs have been included in the medical insurance drug list.
In the field of rare diseases, according to the "2022 Work Plan for the Adjustment of the National Basic Medical Insurance, Industrial Injury Insurance, and Maternity Insurance Drug Catalog", this year has highlighted the attention paid to the treatment of rare diseases and children's medication.
Among the local pharmaceutical companies, Stuuximab for injection from Baiji Shenzhou and Inelizumab injection authorized by Hansen are among them.
Among multinational pharmaceutical companies, Takeda Pharmaceutical's Lanalizumab and Veranase for Injection α; Novartis' Orfatomuzumab; GlaxoSmithKline's mepoxyzumab injection and others have been included in the list.
In the 2021 national medical insurance negotiation, negotiators spoke of "every small group should not be abandoned", and the "life-saving drugs" for the treatment of spinal muscular atrophy, a rare disease, were included in the medical insurance, reducing from 700000 yuan for a single injection when it first entered the domestic market to 33000 yuan.
This year, it is still worth looking forward to the surprises that medication for rare diseases will bring.
In the field of tumor treatment, some anti-tumor drugs from domestic and foreign pharmaceutical companies are among them, including Hengrui's Karelizumab for Injection, Junshi's Treprizumab Injection, Baiji Shenzhou's Tirelizumab Injection, and Xinda's Xindiliximab Liquid.
In the field of traditional Chinese patent medicines and simple preparations, many large varieties have also been included in this list. For example, Jieyu Qufan Capsules, Yishen Yangxin Anshen Tablets, Lianyu Burn Cream, Qiruiweishu Capsules, Xuanqi Jiangu Tablets, etc.
On June 8, the National Health Insurance Bureau released the Statistical Bulletin on the Development of National Medical Security in 2021. According to data, since the establishment of the National Health Insurance Bureau in 2018, it has conducted four consecutive negotiations on the access to the medical insurance drug catalog, adding a total of 250 drugs to the catalog through negotiations, with an average price decrease of more than 50%; In 2021, 140 million people were reimbursed for 221 negotiated drugs during the agreement period; Through negotiated price reductions and medical insurance reimbursement, the cumulative burden reduction for patients during the year was 149.49 billion yuan.
According to the arrangement of the National Health Insurance Bureau, it will next organize expert review and other work in accordance with the requirements of the "Interim Measures for the Administration of Medication in Basic Medical Insurance" and the "Work Plan for the Adjustment of the National Basic Medical Insurance, Industrial Injury Insurance, and Maternity Insurance Drug Catalog in 2022".
Relevant enterprises can pay attention to the "2022 National Medical Insurance Drug Catalog Adjustment and Application Module" on the national medical insurance service platform, and timely obtain relevant review, negotiation, agreement, and other information from the National Medical Insurance Bureau.
Attachment:
Reference source: http://www.nhsa.gov.cn/art/2022/9/9/art_104_9053.html

