9F, Zhongrui Jumei Building, 68 Jiuzhang Road, Suzhou Industrial Park, Jiangsu Province
Medical information
Nature | Experiments between men and women yield very different results! Experimental mice prefer women and hate men, the reason is so surprising...
The failure to replicate experimental results between different laboratories, and sometimes even within the same laboratory, may be due to unrecognized experimental variables that were not properly controlled. The sex of the human experimenter is rarely considered as a biological variable that can affect experimental results and is often not considered in the design, factored in the statistical analysis, or reported in the experimental methods.
On August 30, 2022, the team of Professor Todd D. Gould of the University of Maryland published a research paper titled "Experimenters' sex modulates mouse behaviors and neural responses to ketamine via corticotropin releasing factor" online in Nature Neuroscience. The study found that the gender of human experimenters affects the behavior and response of mice after administration of the fast-acting antidepressant ketamine and its bioactive metabolite (2R,6R)-hydroxynorketamine. Mice showed aversion to the odor of male experimenters, preference for the odor of female experimenters, and showed higher susceptibility to stress when handled by male experimenters.
This aversion and stress susceptibility induced by the odor of a male experimenter is mediated by activation of entorhinal cortical corticotropin-releasing factor (CRF) neurons projecting to hippocampal region CA1. Exposure to the odor of a male experimenter prior to ketamine administration activates entorhinal cortical CRF neurons projecting to CA1, and activation of this CRF pathway mediates the antidepressant-like effects of ketamine in vivo and in vitro. A better understanding of the specific and quantitative contribution of human experimenter sex to findings in rodents may improve reproducibility between studies and, as we have shown, reveal biological and pharmacological mechanisms.
The failure to replicate experimental results between different laboratories, and sometimes even within the same laboratory, may be due to unidentified experimental variables that were not appropriately controlled. The sex of the human experimenter is rarely considered as a biological variable that can influence experimental outcomes and is often not considered in design, factored into statistical analyses, or reported in experimental methods. However, there is evidence that the ability of rodents to discriminate the sex of human experimenters can have measurable effects on their behavior and/or biological responses. For example, exposure to the odor of a male experimenter (but not that of a female experimenter) has been shown to increase anxiety-related behaviors and stress-induced analgesia. Here, the authors investigated the role of experimenter sex in stress-induced maladaptive behaviors and how the odor of male versus female experimenters influences rodent biobehavioral responses to pharmacological antidepressant treatment.
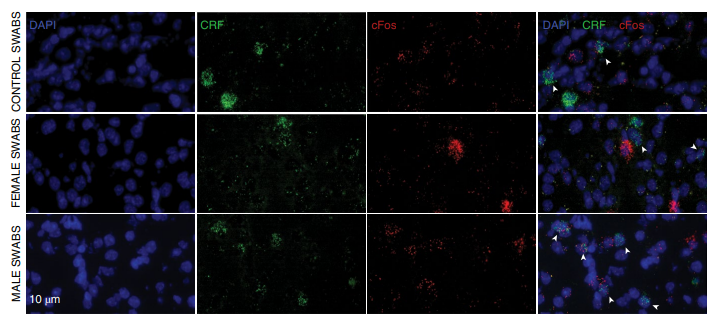
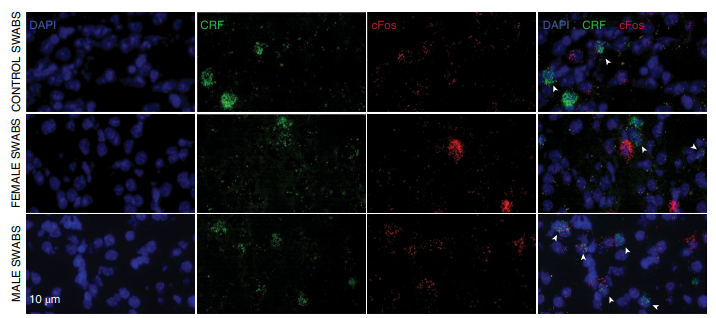
CRF-positive EC cells mediate aversion to the odor of male experimenters (Image from Nature Neuroscience)
The data showed that exposure to the scent of male and female experimenters elicited different behavioral responses and altered the mice's response to antidepressant doses of ketamine (KET). The results are consistent with earlier findings by Sorge et al. that exposure to the scent of a male experimenter led to increased corticosterone levels, stress-induced analgesia, and anxiety-like behaviors in mice. Furthermore, the study showed that the mice's aversion to male odors was due to the activation of CRF-expressing neurons projecting from the EC (entorhinal cortex) to the CA1, and that this pathway underlies the mice's different behavioral responses to male and female experimenters.
The data also revealed that the sex of the experimenter not only affects the basal behavior of mice, but also their response to pharmacological treatment. This finding allowed the authors to deeply dissect the mechanism of ketamine's antidepressant-like effects. In short, exposure of mice to male odor before ketamine administration activated CRF cells in the entorhinal cortex that project to CA1, thereby promoting the regulation of this pathway on ketamine's antidepressant-related effects, both in vivo and in vitro.
The study found that acute ketamine/(2R,6R)-hydroxynorketamine ((2R,6R)-HNK) combined with a CRF receptor agonist may be a novel approach for treating mood disorders. The study also highlights the importance of human experimenter sex on the reproducibility and interpretation of experimental results within and between laboratories. In this study, the stress response induced by the male experimenter resulted in the expected results (antidepressant-like effects of ketamine); it is possible that in other studies, female experimenters are more likely to obtain historically expected results. For example, it has been previously reported that exposure to male experimenters results in stress-induced analgesia, which attenuates the expected pain response in mice and rats. Many other factors may affect behavioral results or influence the potential effect of experimenter sex on experimental results. These factors include, but are not limited to, ventilated cages versus open air cages, mice bred locally versus purchased from commercial suppliers, overall stress exposure within the facility, the circadian cycle of animal testing, unique handling procedures, strains and substrains of mice, experimenter stress level, experimenter diet (meat eaters versus plant-based diet), and hormonal status of the experimenter and/or animals. Controlling these factors and understanding how they specifically and quantitatively affect different experimental outcomes can not only reduce heterogeneity between studies but, equally importantly, uncover new biological mechanisms to advance scientific knowledge and drug discovery.
Original link: https://doi.org/10.1038/s41593-022-01146-x Article source: iNature