9F, Zhongrui Jumei Building, 68 Jiuzhang Road, Suzhou Industrial Park, Jiangsu Province
Medical information
Top HIV research team COVID-19 heavy research: repeated emergence of a rare antibody prompts vaccine clues
The entry of COVID-19 into cells depends on the binding domain (RBD) of the virus's S protein and human cell receptor. Although there is no COVID-19 vaccine, antibodies are crucial to the global epidemic resistance. At present, a top AIDS research team has extracted and identified antibodies from the plasma of COVID-19 rehabilitation patients, and found high and active, rare but recurrent RBD specific antibodies. This suggests that vaccines developed based on this antibody may be generally effective for the general public. The above findings come from the COVID-19 Antibody Heavy Weight Research: Convergent Antibody Responses to SARS-CoV-2 Effect in Convergent Individuals published by Rockefeller University, California Institute of Technology, Howard Hughes Medical Research Institute and Chan Zuckerberg Biohub on the medical preprint website bioRxiv. The paper was published on May 15th local time, and the corresponding authors include Michel Nussenzweig, director of the Molecular Immunology Laboratory at Rockefeller University in the United States, and Professor Paul Bieniasz

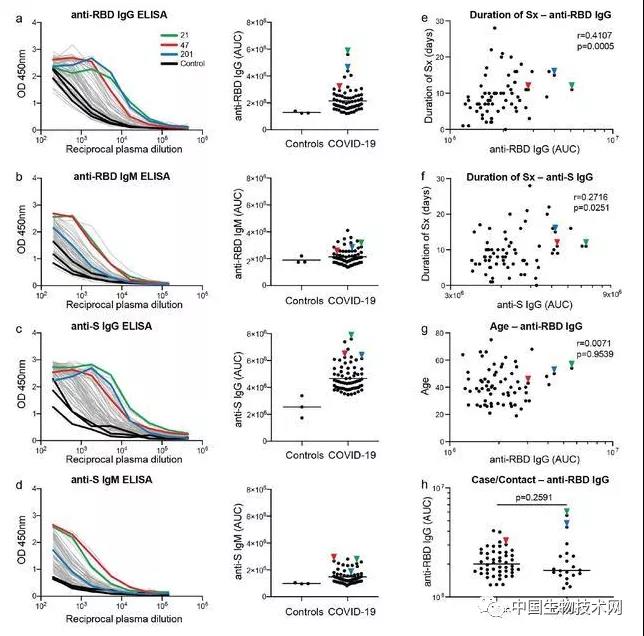
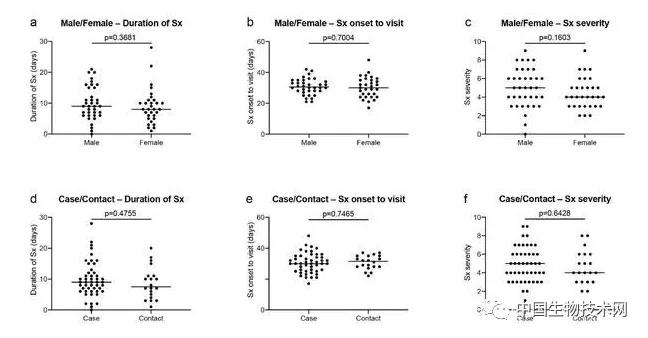
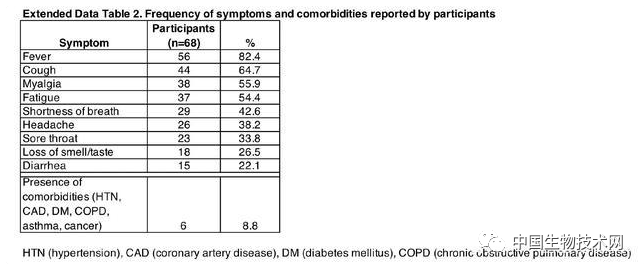
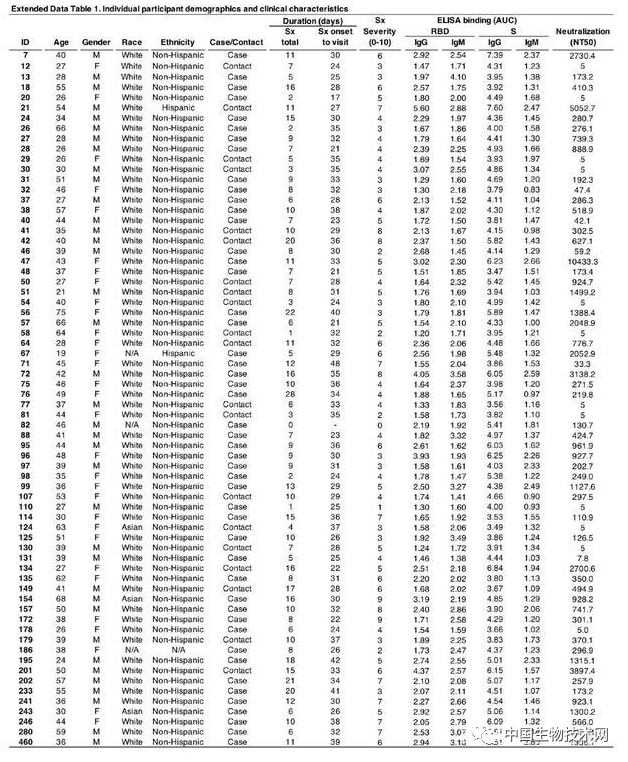
Professor Michel Nussenzweig (left), Professor Paul Bieniasz (right), as a top scholar in the field of AIDS, Nussenzweig's research focuses on the molecular mechanism of the innate and adaptive response of the immune system, and the research methods combine biochemistry, molecular biology and genetics. He focuses on B lymphocytes and HIV-1 antibody research. In response to the COVID-19 outbreak, Nussenzweig has extended its research to SARS-CoV-2 and isolated and identified highly effective neutralizing antibodies from convalescent patients. Another corresponding author, Professor Paul Bieniasz, is also a top scholar in the field of AIDS. His research attempts to define how host genes affect viral replication, focusing on human and primate immunodeficiency viruses. Its laboratory is dedicated to characterizing the host functions that viruses mimic, manipulate, and otherwise utilize, as well as the characteristics of the evolution of defense cells against viral infection. On May 13 local time, Mike Ryan, head of the Health Emergency Project of the World Health Organization (WHO), said that COVID-19 may become a long-term problem. It is difficult to predict when the virus can be defeated. It may become an epidemic virus that will never disappear. Ryan took AIDS as an example, pointing out that although HIV has not disappeared, human beings have found treatment and prevention methods, and people are no longer afraid of AIDS as before. Appendix: Research methods: The S protein of COVID-19 is responsible for binding to human receptors for invasion, which is usually an important target for drug development. During infection, S protein is cleaved by host proteases such as TMPRSS2 into N-terminal S1 subunits and C-terminal S2 subunits, which mediate receptor binding and membrane fusion, respectively. Among them, S1 contains the N-terminal domain (NTD) and receptor binding domain (RBD), which are crucial in determining tissue tropism and host range. When a virus invades the human body, RBD binds to the human receptor ACE2 (angiotensin converting enzyme 2). However, at present, little is known about the human antibody response to SARS-CoV-2. In this study, the research team reported 68 COVID-19 convalescents who were not hospitalized. The distribution of the semi maximum neutralization titer range of plasma collected by these convalescents 30 days after the onset of symptoms varies from undetectable (18% of the sample) to below 1:1000 (78% of the sample), with only 3% of convalescents exceeding 1:5000. Antibody cloning shows the expanded cloning of RBD specific memory B cells (antibodies that express closely related antibodies) in different individuals. Although the plasma titer is low, the semi inhibitory concentration of antibodies targeting unique epitopes on RBD can be neutralized at levels as low as ng/mL. As a result, most individuals recovering from COVID-19 without hospitalization do not contain high levels of neutralizing activity in their restorative plasma. However, rare but recurrent RBD specific antibodies with effective antiviral activity were found in all tested individuals, indicating that vaccines developed based on such antibodies may be generally effective. Between April 1 and April 17, 2020, 73 eligible participants entered the study. Among them, 48 (65.8%) were COVID-19 patients confirmed by RT-PCR (hereinafter referred to as "cases"), and 25 (34.2%) were close contacts of diagnosed cases (hereinafter referred to as "contacts"). Five close contacts without symptoms were excluded from further analysis. At the time of sample collection, these 68 people had no symptoms for at least 14 days. Only one asymptomatic person who tested positive for SARS-CoV-2 nucleic acid had an average onset time of approximately 30 days (17 to 48 days) before sample collection for the other 67 participants. In this cohort, symptoms persisted for an average of 10 days (0-28 days) and were not hospitalized. The most common symptoms are fever (82.4%), cough (64.7%), myalgia (55.9%), and fatigue (54.4%). Complications are rare (8.8%). There were no significant differences between sexes, between cases and contacts, between the duration or severity of symptoms, or between the onset of symptoms and the time taken to collect samples. Of the tested plasma samples, 88% and 66% showed RBD specific IgG and IgM antibodies (at least 2 standard deviations higher than the control group). However, the IgG and IgM antibody responses to trimeric S protein in plasma samples were only 40% and 21% (higher than at least 2 standard deviations in the control group). There were no significant differences between IgG and IgM antibody levels and how long the sample was collected after onset, age, gender, case, or contact. In contrast, the binding of IgG antibodies to RBD and S proteins is directly related to the duration of symptoms, but the binding of IgM antibodies to RBD and S proteins is not related to the duration of symptoms. Finally, there is no significant gender difference in antibody levels against S protein, but the effectiveness of IgG binding antibodies against RBD in women is lower than in men
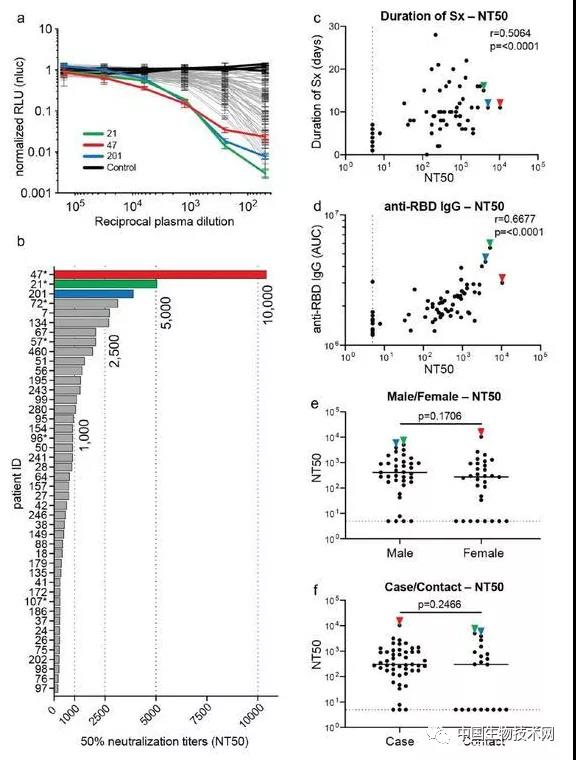
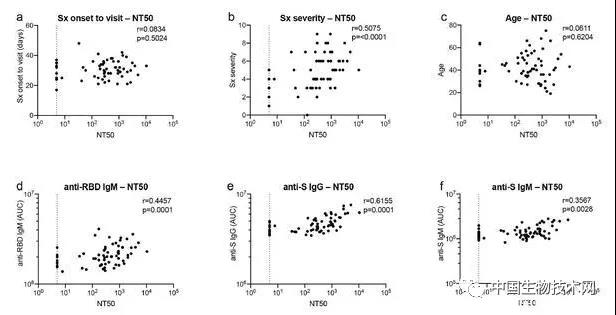
To measure the neutralizing activity of restorative plasma, the researchers used a pseudoviral assay using HIV-1 based viral particles carrying the nanoluciferase reporter gene and SARS-CoV-2S protein. The overall level of neutralization activity in a cohort measured by the semi maximum neutralization titer (NT50) is typically low, with undetectable levels of 18% and below 1000 levels of 78%. The geometric average of NT50 is 212 (arithmetic average=850), and only 2 people have NT50 above 5000. Studies suggest that neutralizing activity is related to the duration and severity of symptoms, but not to sample collection time related to symptoms, age, gender, or onset of case/contact status. It is worth noting that the level of IgG antibodies binding to RBD and S is closely related to NT50
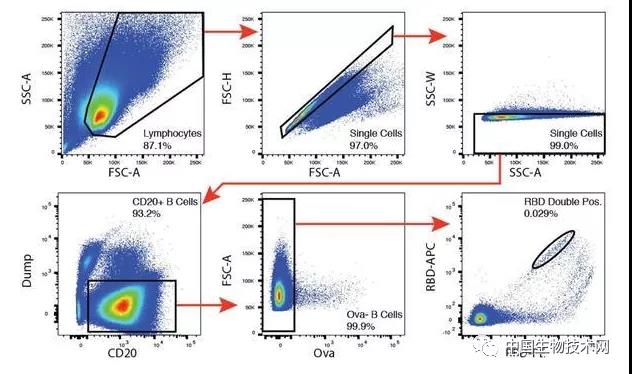
In order to determine the properties of antibodies triggered by SARS coronavirus type 2 infection, the researchers used flow cytometry to isolate B lymphocytes with binding RBD company receptors from the blood of 6 participants, including 2 patients with optimal antibody neutralizing activity. The frequency of antigen specific B cells is determined by their ability to bind to phycoerythrin (PE) and BV711 labeled RBD Corporation. During the recovery period from coronavirus disease in 2019, circulating B cells ranged from 0.07 to 0.005%, but were not detectable in the control group. The researchers obtained 534 pairs of IgG antibody heavy and light chain (high and ice) sequences from a single RBD company bound B cell of 6 convalescent individuals through reverse transcription and polymerase chain reaction. Compared to the human antibody library, several of the high and ice genes significantly exceed the standard. The average number of nucleotide mutations in Gao and Bing's five genes is 4.2 and 2.8, respectively, which is lower than antibodies cloned from individuals with chronic infections, such as hepatitis B or HIV-1, and similar to antibodies derived from primary malaria infection or non antigen enriched circulating IgG antibody memory cells.
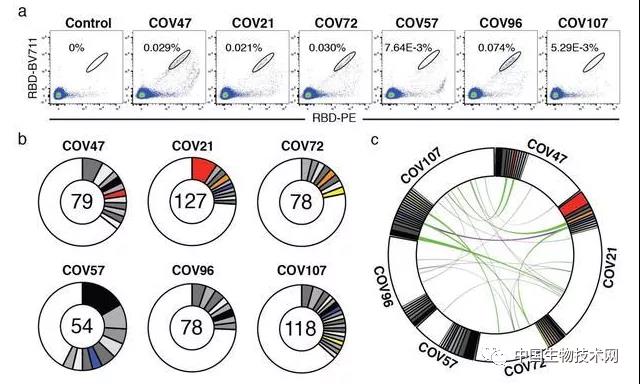
Like other human pathogens, amplified clones of antigen binding B cells were present in all individuals tested for 2019 coronavirus disease. Overall, 32.2% of the restored high and ice sequences come from cloned amplified B cells (range: 21.8-57.4%). Antibodies that share specific combinations of high voltage and ice vapor genes in different individuals account for 14% of all cloned sequences. It is worth noting that some antibodies found in different individuals have almost the same amino acid sequence. For example, the amino acid sequences of clonal antibodies with IGHV1-58/IGKV3-20 and IGHV3-30-3/IGKV1-39 types found repeatedly in different individuals have identities of up to 99% and 92%. The researchers concluded that the IgG antibody memory response to severe acute respiratory syndrome coronavirus 2RBD is highly enriched in the cyclically cloned antibody sequence.
To examine the binding characteristics of anti SARS coronavirus type 2 antibodies, the researchers expressed 34 representative antibodies, of which 24 were from clones and 10 were from monomers (found in the plasma of three participants). Enzyme linked immunosorbent assay analysis showed that 94% (32 of 34 antibodies) were bound to severe acute respiratory syndrome coronavirus 2RBD at a semi maximum effective concentration of 6.6 ng/mL (December 50),. In order to determine whether these antibodies have neutralizing activity, the researchers conducted tests against the severe acute respiratory syndrome coronavirus type 2 backbone pseudovirus. Among the 32 RBD company binding antibodies tested, 20 were found to be capable of small neutralization at a semi maximum inhibitory concentration (IC50) of the order of nanograms per milliliter, with concentrations ranging from 4.4 to 709 nanograms per milliliter. Effective neutralizing antibodies were found in individuals, independent of plasma NT50 type. For example, C002 and C121 were obtained from individual coronavirus 21 and coronavirus 107 with plasma NT50 values of 5053 and 298, respectively, with IC50 values of 8.9 and 6.7 ng/mL. Finally, cloning of antibodies that share high voltage and ice vapor genes is one of the best neutralizing agents, such as antibody C002 with IGHV3-30/IGKV1-39, both of which have the best plasma neutralizing activity. The researchers concluded that even people with moderate plasma neutralizing activity have rare IgG antibody memory B cells that produce effective neutralizing antibodies against SARS coronavirus type 2. In order to determine whether human anti SARS coronavirus type 2 monoclonal antibodies with neutralizing activity can bind to different domains on RBD Corporation, the researchers conducted a double layer interference measurement experiment in which the pre formed antibody RBD Corporation immune complex was exposed to a second monoclonal antibody. The antibodies tested included 2 groups, while C002 and 2005 bound to the pre formed C121-RBD company complex, while C104, C110, and page 119 did not. The conclusion is that, like severe acute respiratory syndrome coronavirus, there are at least 2 different neutralizing epitopes on the RBD company of SARS coronavirus type 2. Through single cell antibody cloning, individuals obtain human monoclonal antibodies that have neutralizing activity against pathogens such as viruses and parasites in natural infections. Several effective methods of protection and treatment have been shown in model biology and early clinical studies, but only one antiviral monoclonal drug is currently in clinical use. Antibodies are relatively expensive and more difficult to produce compared to small molecule drugs. However, they differ from drugs in that they can interact with Fc on host immune cells γ The constant domain of receptor binding is involved in the host immune system. These interactions can enhance immunity and help clear pathogens or infected cells, but they can also lead to increased dengue and coronavirus infections. This problem hinders the development of dengue vaccine, but does not interfere with the clinical application of effective neutralizing antibodies, which can be modified to prevent Fc γ Receptors interact and maintain protection against viral pathogens. Antibodies are essential elements in most vaccines and may become a key component of an effective vaccine against SARS coronavirus type 2. Observations have shown that the plasma neutralizing activity of most convalescent patients is low, but antibodies with effective neutralizing activity against the recurrence of severe acute respiratory syndrome coronavirus 2RBD can be found in patients with abnormal plasma neutralizing activity, indicating that humans have an inherent ability to produce antibodies against SARS coronavirus type 2 that can be effectively neutralized by anti RBD companies. Therefore, vaccines that specifically and effectively induce antibodies targeting severe acute respiratory syndrome coronavirus 2RBD may be particularly effective. Demographic and clinical characteristics of participants:</ P>

